
GAMP 5: It is a Risk-Based Approach to Compliant GxP Computerized Systems sets out guidelines for the risk-based evaluation of computer system accuracy, which is different for each system based on whether it is intended for use or not.
Gamp category 1 examples software#
The GAMP software categories are introduced to provide an initial assessment of validation requirements or deliverables. Category 1: Infrastructure Software including Operating System, Database managers. LIMS, SCADA, CDS, DCS.Ĭategory 2 has been removed from GAMP 4 version. GAMP® Software Category Definitions First, let’s define the Software Categories within the GAMP 5 Guidelines.
Category 3: Non-configurable Software including Commercial of the shelf software (COTS), Laboratory Instrument / Software. Category 1: Infrastructure Software including Operating System, Database managers. In GAMP 4 there were 5 software categories but in GAMP 5 it was revised from 5 categories to 4 categories and mentioned as below: GAMP 5 category 1 software includes tools used for testing or data masking. The Guide facilitates the effective and efficient use of valuable resources by the application of appropriate and proportionate practices, encouraging innovative approaches to managing risk to patient safety, product quality, and data integrity, while supporting benefit to public health.There are 5 software categories as per the International Society for Pharmaceutical Engineering (ISPE). Technological innovation is essential for life sciences industries in providing value to society while also controlling costs and reducing time to market. 
The V-model of the systems engineering process.

A risk assessment must be kept under review in order to: Ensure that agreed working practices continue to be applied. categories, the German V-Modell, a general testing model, and the US government standard. The ISPE GAMP ® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems Second Edition aims to protect patient safety, product quality, and data integrity by facilitating and encouraging the achievement of computerized systems that are effective, reliable, and of high quality. Keep them safely stored to support audits. GAMP ® guidance does not define a prescriptive method or a standard, but rather provides pragmatic guidance, approaches, and tools for the practitioner. Promotes a system life cycle approach based on good practice.Establishes a common language and terminology.
Table 4.1 Example of Software Testing Criticality No testing of Category 1.
Facilitates the interpretation of regulatory requirements Based upon the GAMP 4 GxP Priority and the software/hardware category of. GAMP® is an ISPE Community of Practice (CoP). Using the GAMP 5 software categorization, laboratory computerized systems fit into software categories 3 and 4 and to some degree Category 5, although it needs to be noted that Categories 3 to 5 are effectively a continuum with no absolute boundaries. GAMP® adopts a patient-centric risk-based approach that enables innovation while demonstrating compliance with regulatory requirements. Platforms on which computer applications or elements are necessary to operate and manage information technology environments run. It aims to achieve computerized systems that are fit for intended use and meet current regulatory requirements by building upon existing industry good practice in an efficient and effective manner. 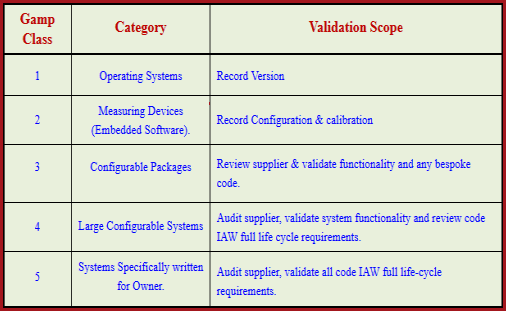
GAMP ® guidance aims to safeguard patient safety, product quality, and data integrity in the use of GxP computerized systems.








 0 kommentar(er)
0 kommentar(er)
